Abstract
Background: Patients with lupus anticoagulant (LA) are at risk for arterial and venous thromboembolic events. Recent work suggests that LA positive patients who experience thrombotic events in different vascular beds constitute distinct subgroups. To risk stratify patients, further work is needed to better characterize predictors for thromboembolic events in these subgroups.
Aims: The aim of this study was to identify baseline characteristics and laboratory parameters associated with the development of arterial or venous thrombotic events.
Methods: Patients with at least 2 previous positive LA tests were serially monitored for thrombotic events within the prospective Vienna Lupus Anticoagulant and Thrombosis Study (LATS). Patients without clinical follow-up were excluded from this analysis. Statistical analysis was performed with RStudio (Version 1.1.442).
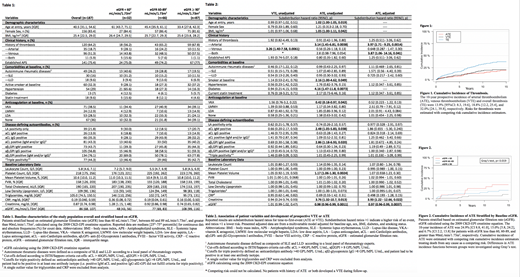
Results: One-hundred-eighty-seven patients were followed (Table 1) for a median of 11.4 years and 1865 follow-up visits (median visit/patient=9). Fifty-seven prospective thrombotic events (TE), including 27 arterial thrombotic events (ATE) and 30 venous thrombotic events (VTE), were observed. This corresponded to 10-year prospective ATE, VTE and overall thrombosis incidences of 13.9% [95%CI: 8.3, 19.6], 18.8% [12.2, 25.4], and 32.0% [24.1, 39.8], respectively. (Figure 1). Thirty-seven of the 57 events occurred in patients with a prior history of thrombosis ("recurrent thrombosis").
In univariable competing risk analysis, age (subdistribution hazard ratio (SHR) = 1.02, 95% CI: 1.00-1.05, p=0.019), body mass index (BMI, 1.05, 1.00-1.11, p=0.042), history of ATE (3.14, 1.45-6.81, p=0.0038), active smoking (2.16, 1.00-4.62, p=0.049), diabetes (4.16, 1.47-11.8, p=0.0073), VKA use at baseline (0.42, 0.18-0.97, p=0.042), aCL IgM positivity (2.48, 1.05-5.83, p=0.038), aβ 2GPI IgM positivity (2.86, 1.18-6.93, p=0.020), mean platelet volume (1.17, 1.06- 1.30, p=0.0024), creatinine (3.76, 1.32- 10.7, p=0.013), and estimate glomerular filtration rate (eGFR, 0.98, 0.96-0.99, p=0.0011) were associated with prospective risk of ATE (Table 2). Conversely, the prospective risk of VTE was univariably associated only with prior history of VTE (3.26, 1.40-7.68, p=0.0061).
After adjusting for traditional arterial thrombotic risk factors (age, sex, BMI, active smoking, diabetes,), history of ATE (SHR = 3.97, 95% CI: 1.71-9.025, p=0.0014), prior history of both ATE and VTE (3.87, 1.06-14.16, p=0.041), creatinine (3.93, 1.22-12.66, p=0.022), and eGFR (CKD-EPI, 0.96, 0.96-0.99, p=0.0037) remained independently associated with prospective risk of ATE (Table 2).
In detail, the 10-year cumulative risk of ATE was 24.9% [95%CI: 8.8, 41.0], 15.0% [5.8, 24.2], and 6.7% [2.2, 13.8] in patients with a baseline eGFR less than 60 mL/min/1.73m 2, between 60 and 89 mL/min/1.73m 2, and greater than or equal to 90 mL/min/1.73m 2, respectively (Gray's test, p=0.019, Figure 2).
Conclusion: Approximately 14% of patients persistently positive for LA experienced an ATE over 10 years. After adjusting for traditional arterial risk factors, decreased renal function was associated with an increased prospective risk of ATE. Notably, decreased renal function was not associated with development of VTE and the association with ATE was also independent of underlying SLE, LLD, or rheumatic disease (data not shown). Clinically, LA positive patients with decreased renal function may represent a subgroup that might benefit from more aggressive anti-thrombotic therapy or anti-thrombotic prophylaxis.
Pabinger: Bayer: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Daiichi Sanchyo: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; NovoNordisk: Consultancy, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal